Liquid crystals can be divided into two subgroups: thermotropic and lyotropic liquid cyrstals. Lyotropic liquid crystals (LLCs) include at least two key components, a solvent and a surfactant. As the solvent helps to stabilize the surfactant molecules, the result can be defined as a “mesophase”, a phase that is in between solid and liquid. The liquid part comes from the physical properties, while the solid part comes from the order of the surfactant molecules.
The Dag Group discovered in 2001 that the solutions containing the precursors and certain metal salts result in LLC mesophases1. Further studies showed that the transition metal salts in these solutions remain in the molten phase, preventing the nucleation of salt crystals from occuring in the liquid crystal phase and thus forming a stable solution2. These studies also indicate that the salts in molten phase act as a solvent and remain in the molten phase due to the confinement effect, in which the solvent molecules reside in 1-2 nm gaps between the micelles.
Another valuable study in this field shows the salts with have high melting point and solubility also form LLC phases with the addition of water molecules (2-5 water molecules per salt). A depression in the melting point of the salts such as alkali salts (e.g., lithium) and alkaline earth salts (e.g, calcium and magnesium) due to the confinement effect (CE)3. Generally, the solubility of these metal salts in surfactants are very low, but exceeding a critical micelle concentration enables the formation of LLC phases, with the salt remaining in the molten phase. This can be extended to two-surfactant systems: one being oligo (ethylene oxide) or Pluronics, and the other a charged surfactant such as SDS or CTAB. It has been observed that the LLC systems containing two surfactants form much more stable micelle assemblies.
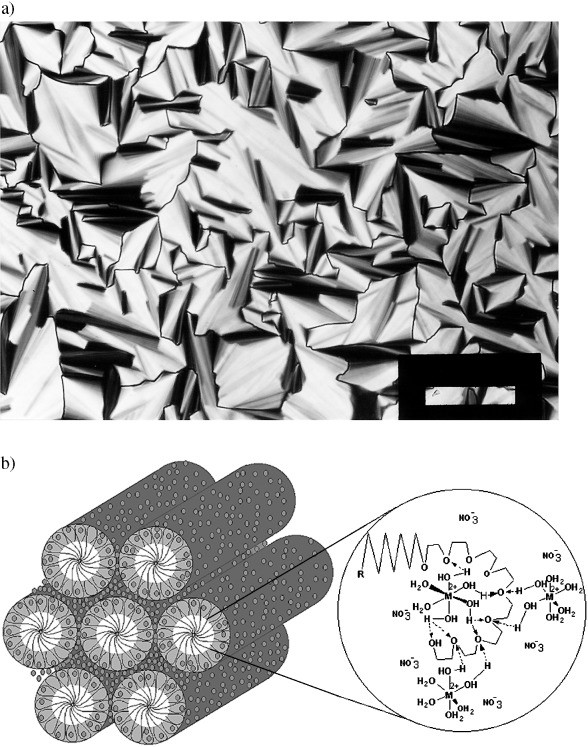
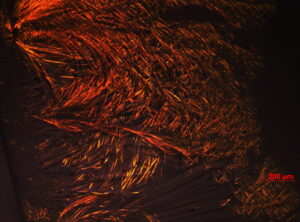
Figure 1. a) A representative POM image of [Cd(H2O)4](NO3)2 in C12H25(CH2CH2O)10OH with a MX2/No mole ratio of 2.0/1 (the scale bar is 200 μm). b) A schematic representation of a hexagonal LC MX2/No structure, the small circles represent [M(H2O)n]2+ and NO3− ions.
References
- Ö. Çelik and Ö. Dag, “A New Lyotropic Liquid Crystalline System: Oligo(ethylene oxide) Surfactants with [M(H2O)n]Xm Transition Metal Complexes,” Angewandte Chemie International Edition, vol. 40, no. 20, pp. 3799–3803, Oct. 2001, doi: https://doi.org/10.1002/1521-3773(20011015)40:20%3C3799::aid-anie3799%3E3.0.co;2-i. ↩︎
- Cemal Albayrak, Necati Özkan, and Ö. Dag, “Origin of Lyotropic Liquid Crystalline Mesophase Formation and Liquid Crystalline to Mesostructured Solid Transformation in the Metal Nitrate Salt−Surfactant Systems,” Langmuir, vol. 27, no. 3, pp. 870–873, Oct. 2010, doi: https://doi.org/10.1021/la1035932. ↩︎
- C. Karakaya, Y. Türker, C. Albayrak, and Ö. Dag, “Assembly of Molten Transition Metal Salt–Surfactant in a Confined Space for the Synthesis of Mesoporous Metal Oxide-Rich Metal Oxide–Silica Thin Films,” Chemistry of Materials, vol. 23, no. 12, pp. 3062–3071, May 2011, doi: https://doi.org/10.1021/cm200932k ↩︎