Mesoporous materials can be characterized by the order of their pores. Generally, ordered mesoporous thin films are synthesized using template methods. The Dag Group employs the soft template methods, which involve the formation of the structure either in solution or in the LLC phase through the aggregation of surfactant molecules. This phase is then transformed into a mesostructured solid by calcination at elevated temperatures or solvent extraction which aims to remove the surfactants.
The early findings from Dag Group on LLCs and the studies mentioned above contributed to the development of the Molten Salt Assembly Method (MASA), which was introduced to the literature by this group in 2011 [4]. The MASA method was first applied to Zn(II) and Cd(II) salts as inorganic species in ethylene oxide (oligo) non-ionic and CTAB (ionic) surfactants.
The MASA method demonstrated that mesoporous silica metal oxide thin films can be synthesized by calcination of mesophases consisting of salt species confined between the silica and surfactant domain in their molten phase [5, Fig. 2].
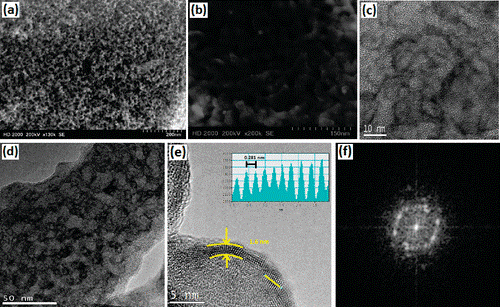
Figure 2. SEM images of meso-SiO2–ZnO-1.14 (a, b). TEM images of meso-SiO2–ZnO-n; n is (c) 0.86, (d)1.14, and (e) 0.57 (inset is yellow line showing spacings between lines), and FFT of a crystalline domain (f).
The Dag Group also demonstrated the synthesis of mesoporous metal titanates, transition metal oxides (MxOy), sulfides (MxSy) and phosphates (M3(PO4)2) using soft templates designed from salt-surfactant LLC mesophases.
These works on mesoporous thin films also aimed to include the evaluation of their performance in electrochemical applications: